Material Informatics for the Development of High-Performance Solid Electrolytes in Rechargeable Batteries
Researchers have developed highly conductive, new organic ionic plastic crystal-based solid electrolytes for use in rechargeable batteries
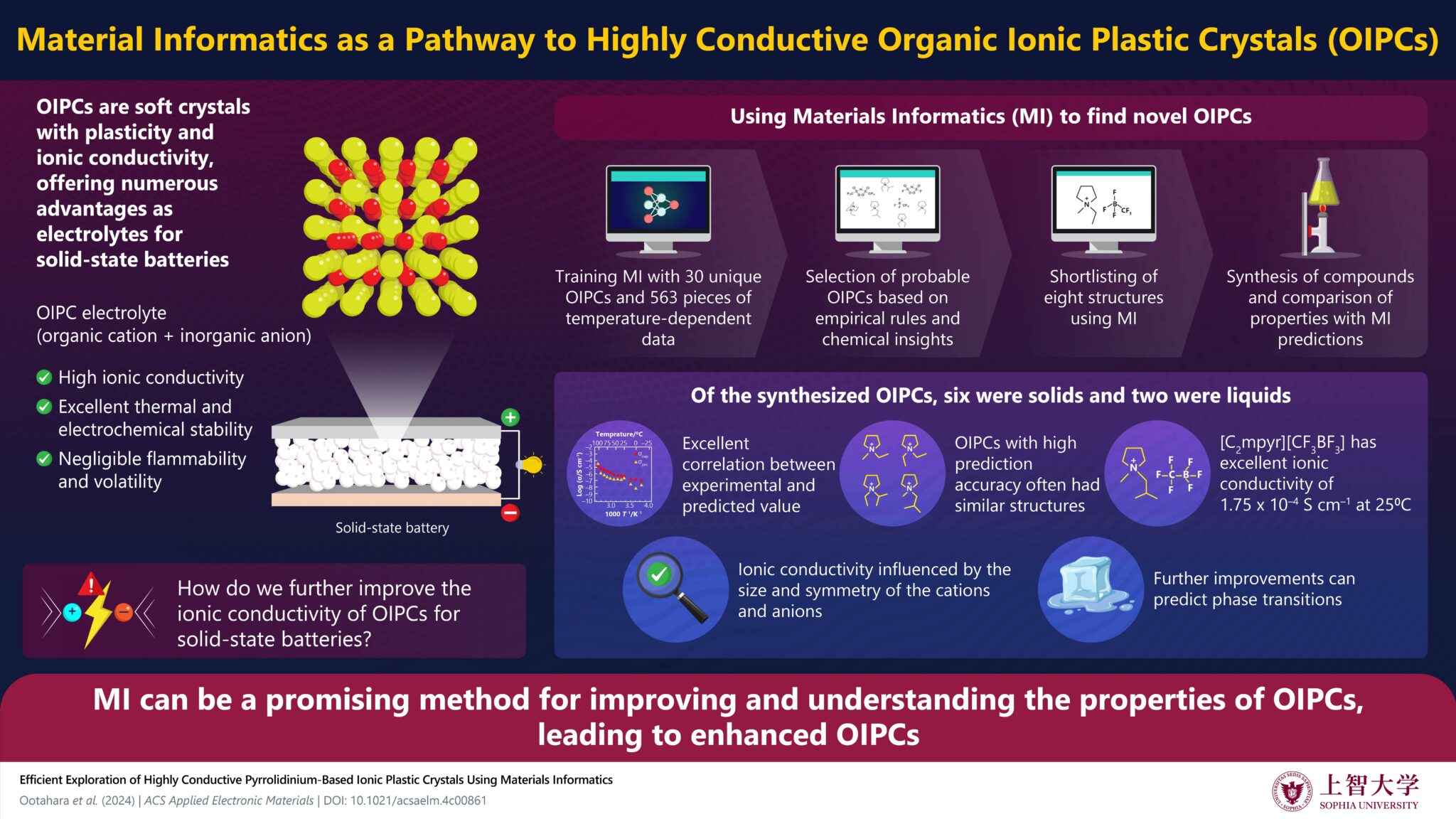
Materials informatics revealed new information on the structure-property relationship in OIPCs, enabling the discovery of new compounds with high ionic conductivities.
Organic ionic solid electrolytes (OIPCs) offer various desirable properties, with high potential as solid electrolytes for all-solid-state rechargeable batteries. However, they still need improvement in their ionic conductivity for practical applications. In a new study, researchers utilized Material Informatics (MI) to develop highly conductive OIPCs.
Incorporating empirical rules and chemical insights into an MI model revealed new information on the structure and ionic conductivity relationship of OIPCs, paving the way for their practical applications.
The surge in the adoption of renewable energy, coupled with the rapid growth of the electric vehicle market in recent years has significantly increased the demand for high-performance, all-solid-state batteries.
Compared to conventional liquid electrolyte-based batteries, solid-state batteries offer higher energy density, improved safety, longer lifespan, and reliable operation over a wide temperature range.
However, there are still challenges to their widespread applications, including low ionic conductivity, high interfacial resistance, and the presence of particle-particle interfaces in the electrolyte, which leads to increased resistance and lower energy density.
Notably, research on high-performance solid electrolytes has primarily focused on inorganic and organic solid electrolytes. While inorganic solid electrolytes transport only lithium ions, organic solid electrolytes allow the migration of anions and other species. However, this leads to side reactions at the electrodes, resulting in reduced capacity and adverse effects, such as decreased battery performance and lifespan.
In contrast, inorganic electrolytes are less prone to side reactions, offering longer battery life and higher performance. Nevertheless, they have their own challenges. For instance, oxide-type inorganic solid electrolytes suffer from reduced stability and require high-temperature sintering, whereas sulfide-type electrolytes react with atmospheric moisture, generating toxic hydrogen sulfide gas.
To address these issues, researchers from Japan undertook a new study by turning their focus toward organic ionic plastic crystals (OIPCs). OIPCs consist of an organic cation and a suitable inorganic anion together with the lithium salt of the same anion.
Being entirely composed of ions, these materials offer high ionic conductivity, high stability, and negligible flammability, making them highly suitable as solid electrolytes for batteries. A notable feature of OIPCs is their phase transition between the solid crystalline phase and the liquid phase, called the plastic crystal phase. Despite these advantages, for practical applications, OIPCs still need higher ionic conductivity.
In the study, the research team, led by Professor Masahiro Yoshizawa-Fujita from the Department of Materials and Life Sciences at Sophia University, along with Takuto Ootahara and Morgan L. Thomas, also from Sophia University, and Kan Hatakeyama-Sato from Tokyo Institute of Technology, utilized Material Informatics (MI) to explore highly conductive OIPCs.
“MI leverages informational science, such as statistical science and machine learning, for efficient material development. In this study, we explored OIPCs by combining empirical rules and a machine learning-based MI model,” explains Prof. Yoshizawa-Fujita. Their findings were made available online on July 29, 2024, and published in Volume 6, Issue 8 of the ACS Applied Electronic Materials on August 27, 2024.
First, the researchers created a training dataset using chemical structures and conductivity data from OIPC-related literature and verified the prediction accuracy of the MI model on two test compounds. The validation results showed that the prediction accuracy improves when the training data includes similar chemical structures.
Therefore, the researchers selected pyrrolidinium cations, which were well-represented in the training data, as candidate substances. Furthermore, based on empirical rules from previous studies on enhancing ionic conductivity in pyrrolidinium cation-based OIPCs, they used MI to further narrow down the candidate substances.
As a result, the team successfully synthesized eight new compounds, including six OIPCs and two ionic liquids. Among these, one compound exhibited excellent ionic conductivity of 1.75 × 10-4 S cm-1 at 25°C, which is among the highest reported value to date.
Notably, the MI results also revealed new insights into the relationship between ionic radius and ionic conductivity of OIPCs. Conventional empirical rules suggest a lower ionic radius to ionic conductivity ratio is desirable. However, the newly synthesized compounds indicate that an optimal value exists.
Additionally, the MI model predicted discontinuous changes in the OIPC structure, suggesting that further improvements in prediction accuracy can also enable the prediction of phase transitions.
Explaining the potential benefits of the new OIPCs, Prof. Yoshizawa-Fujita says, “The development of high-performance solid electrolytes will increase the safety of rechargeable batteries, as there will no longer be a concern about liquid leakage. Also, it will increase the energy density of these batteries, making devices equipped with batteries lighter and more compact. For example, OIPC-based rechargeable batteries can increase the range of electric vehicles and promote their widespread adoption.”
Overall, these findings demonstrate the potential of MI to advance our understanding of OIPCs, paving the way for the development of safer, high-performance, and next-generation rechargeable batteries.
Reference
- Title of original paper
Efficient Exploration of Highly Conductive Pyrrolidinium-Based Ionic Plastic Crystals Using Materials Informatics
- Journal
ACS Applied Electronic Materials
- Authors
Takuto Ootahara1, Kan Hatakeyama-Sato2, Morgan L. Thomas1,2, Yuko Takeoka1, Masahiro Rikukawa1, and Masahiro Yoshizawa-Fujita1
- Affiliations
1Department of Materials and Life Sciences, Sophia University, Japan
2Materials Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, Japan
3Graduate School of Science and Technology, Keio University, Japan
About Professor Masahiro Yoshizawa-Fujita
Dr. Masahiro Yoshizawa-Fujita is currently a Professor at the Department of Materials and Life Sciences, Sophia University. He received his Ph.D. degree (2002) from Tokyo University of Agriculture and Technology, Japan.
After completing his Ph.D. studies, he was awarded a Research Fellowship for Young Scientists by the Japan Society for the Promotion of Science (JSPS) to pursue postdoctoral research at the same university. He then spent two years as a postdoctoral research fellow (Discovery-Project) at Monash University, Australia.
In 2006, he joined Sophia University as an Assistant Professor and was promoted to the position of Professor in 2019. He has published over 170 articles, which have received nearly 12,000 citations. His recent research activities focus on designing organic salts, including ionic liquids, ionic plastic crystals, zwitterions, etc., particularly for battery research and biomass processing.
Funding Information
This study was supported by JSPS KAKENHI (19K05604, 22K19072, and 23H02072), JSPS Bilateral Program (JPJSBP120199977), JST-GteX (JPMJGX23S3), and a Sophia University Special Grant for Academic Research.
Media Contact
Office of Public Relations, Sophia University
sophiapr-co@sophia.ac.jp